Influenza Vaccine Type
8 rows Quadrivalent Influenza vaccine. Available influenza vaccines include including quadrivalent inactivated influenza vaccines IIV4s recombinant influenza vaccine RIV4 or live attenuated influenza vaccine LAIV4.

Flu Season Important Facts Ppt Video Online Download
In most countries this is still the case and the.
Influenza vaccine type. Trivalent flu vaccines protect against three strains of the virus. During the 202122 influenza season the following types of vaccines are expected to be available. All commercially available flu vaccines in the United States are made by private sector manufacturers.
Influenza A two subtypes Influenza B two lineages AH1N1pdm09 AH3N2 Washington Phuket Quadrivalent vaccines QIV protect against viruses for both influenza. People aged 6 months to. Who should have the vaccine.
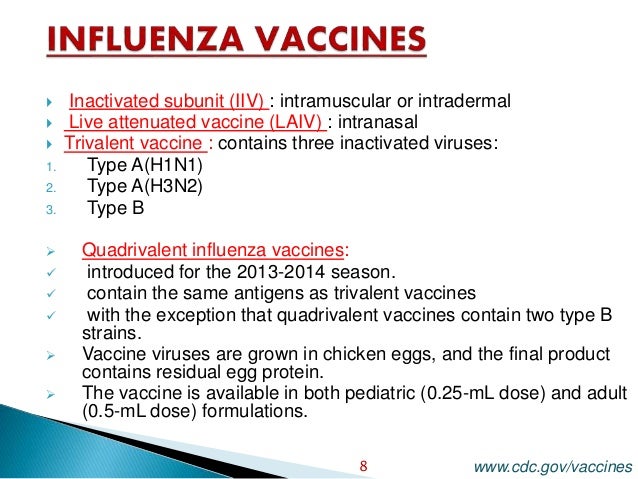
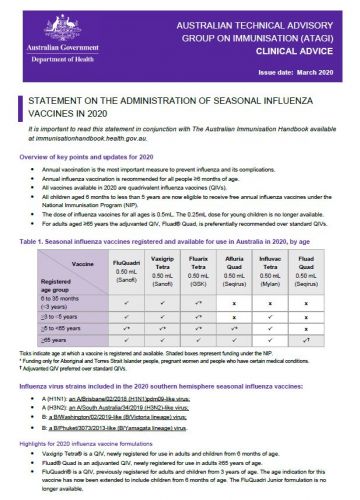
In the 2020-21 season three different types of inactivated flu vaccine will be offered in the UK as well as the nasal flu vaccine. QIVe standard egg-grown quadrivalent. All live attenuated influenza vaccines currently available are quadrivalent combination vaccines containing two influenza A strains H1N1 and H3N2 subtypes and two influenza B strains Victoria and Yamagata lineages as per WHO recommendations.
No preference is expressed for any influenza vaccine over another. Previously all flu vaccines protected against three influenza viruses. Licensed influenza vaccines include inactivated or live-attenuated influenza type A and B viruses with three or four subtypes per vaccine.
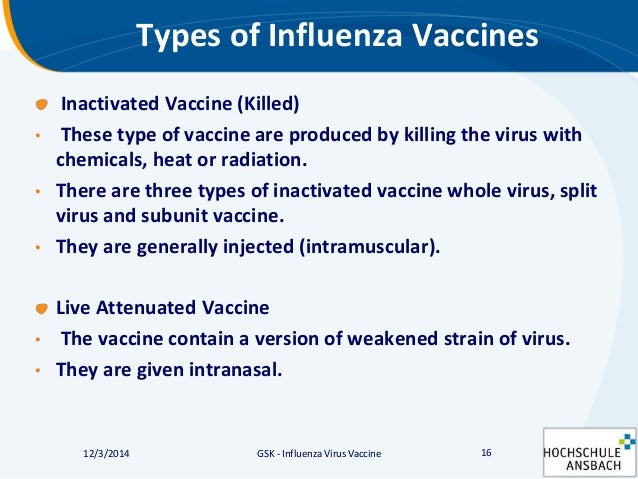
New influenza vaccine developments. Two types of influenza vaccine are available an inactivated killed preparation that is injected and an attenuated influenza vaccine normally delivered nasally. Seasonal influenza vaccination strategies.
The vaccines used for influenza are made up of three different influenza viruses like influenza type A with H3N2 virus strain influenza type A with H1N1 virus strain and influenza type B virus strain. In subunit vaccines HA and NA. Below are links to more information about the different type of flu vaccines available.
Timing of influenza vaccination. Risk groups for severe influenza. In addition a vectored vaccine often enables delivery of the vaccine to sites of inductive immunity such as the respiratory tract enabling protection from influenza.
Influenza A H1N1 influenza A H3N2 an influenza B virus. Type of influenza vaccine. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials.
Currently there are two influenza A subtypes and two influenza B lineages that are circulating globally. Today FluMist and some traditional flu. For the United States there are three different influenza vaccine production technologies approved by the US.
LAIV and QIVc are not licensed for children under 2 years of age. Two types of influenza vaccine are widely available. When the vaccine is injected into the body cells read the genes and make virus proteins which.
EMA offers a specific type of marketing authorisation to allow a vaccine to be developed and authorised before an influenza pandemic. Immunity following influenza disease and administration of influenza vaccines. Quadrivalent flu vaccines protect against four different flu viruses.
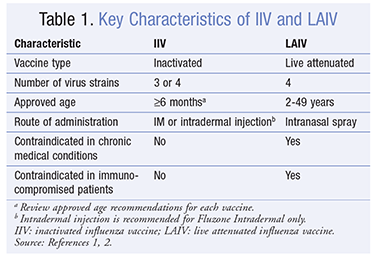
Traditionally influenza vaccines both IIV and LAIV have been produced to protect against 3 different seasonal influenza viruses also called trivalent vaccines. Quadrivalent QIV influenza vaccines There are two main types of influenza that cause disease in humans. People aged 65 years should receive adjuvanted influenza vaccine but may receive a standard influenza vaccine if the.
The vaccines are tested to determine whether. Egg-based flu vaccine cell-based flu vaccine and. At risk children aged from 6 months to less than 2 years.
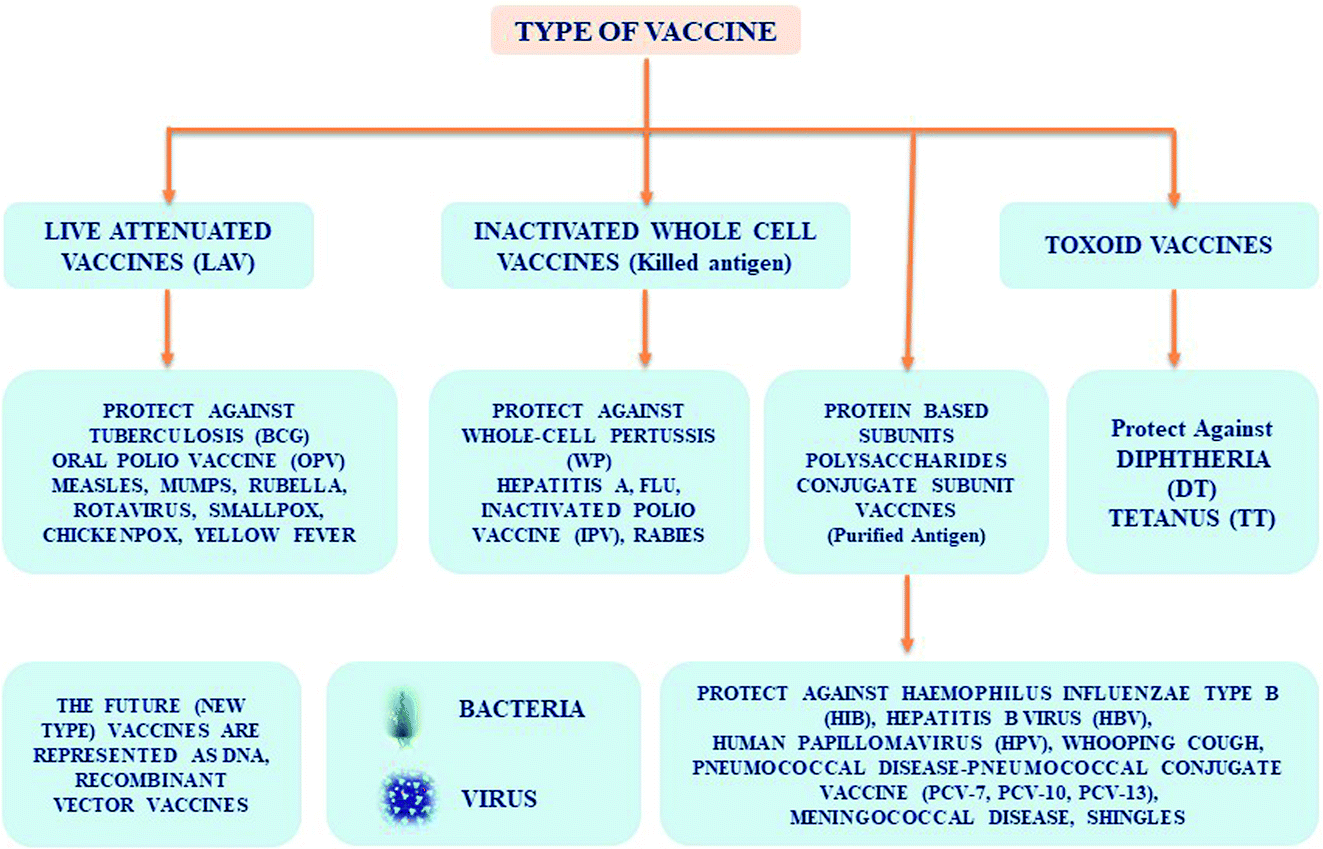
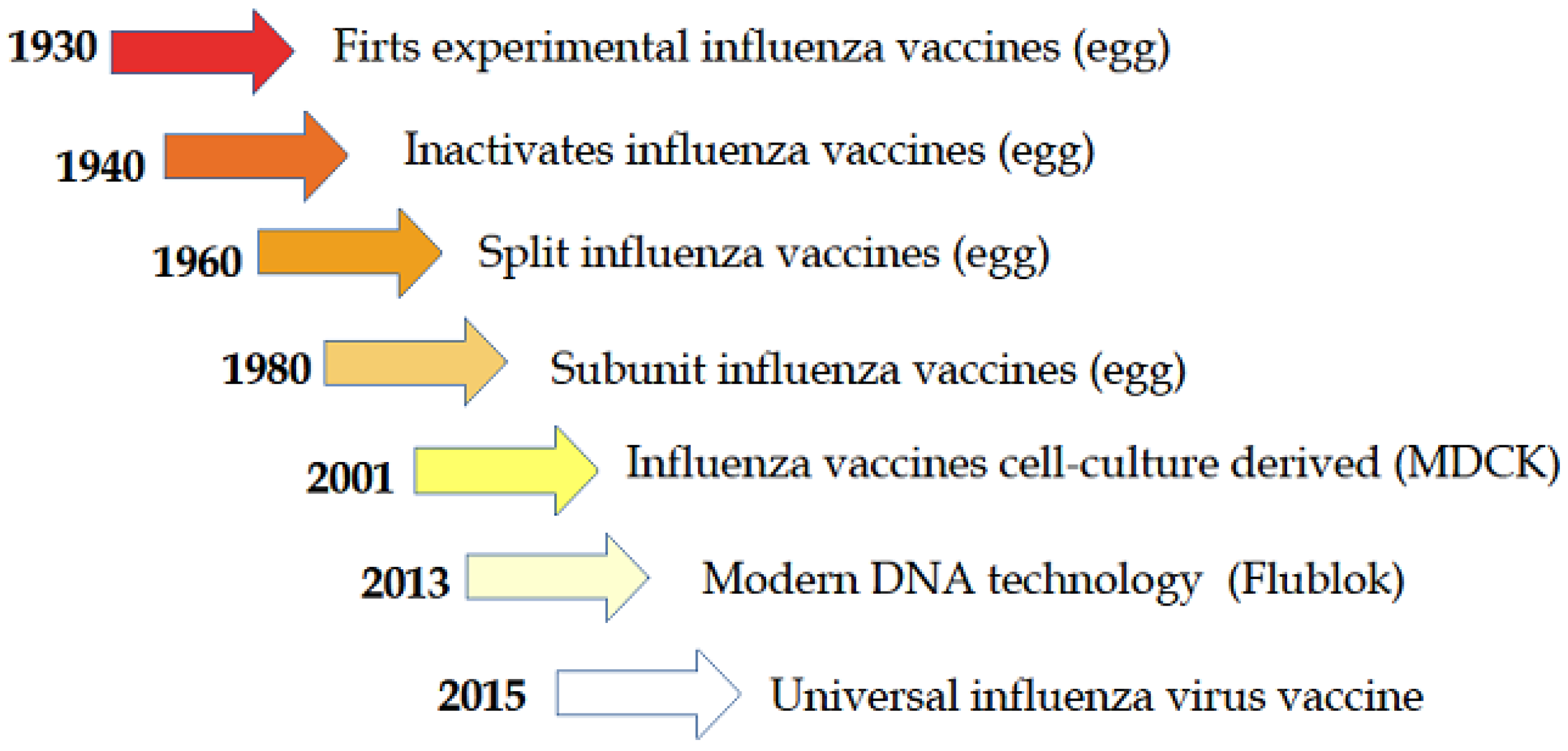
Inactivated influenza vaccines IIV and live attenuated influenza vaccines LAIV. Various influenza vaccines including inactivated split- and subunit-type recombinant and live attenuated vaccines have been developed since the 1930s when it was discovered that influenza viruses could be cultivated in embryonated eggs. However the protection rate offered by these vaccines is rather low especially in very young children and the elderly.
Food and Drug Administration FDA external icon. In this review we describe the history of influenza vaccine development the immune responses induced by the vaccines. Different manufacturers use different production technologies but all flu vaccines meet FDA safety and effectiveness requirements.
The type of vaccine used depends on the persons age. Live-attenuated influenza vaccines LAIV are delivered as nasal spray. Increase in prevalence of influenza epidemics and seasonal outbreaks is likely to expand the product sales during the projected period.
Recombinant virus-vectored vaccines are an appealing alternative to classical inactivated vaccines because virus vectors enable native expression of influenza antigens even from virulent influenza viruses while expressed in the context of the vector that can improve immunogenicity. Types of seasonal influenza vaccine. 27 rows Live Attenuated Influenza Vaccine LAIVNasal Spray Vaccine.
One Influenza A H3N2 virus one Influenza A virus and one Influenza B virus. Hib disease Haemophilus influenzae type b HPV Human Papillomavirus Influenza flu Measles. Inactivated influenza vaccines IIV4s recombinant influenza vaccine RIV4 and live attenuated influenza vaccine LAIV4.
Only IIVs are licensed for children younger than age two years. In split virus vaccines the virus has been disrupted by a detergent. There are three types of inactivated vaccines the whole virus vaccines split virus vaccines and subunit vaccines.
Flu shots are vaccines given with a needle usually in the arm. A DNA vaccine contains a small circular piece of DNA called a plasmid that includes genes that code for proteins of a flu virus. Regular standard-dose trivalent shots.
List of Influenza Virus Vaccine Quadrivalent Types A and Types B. In 202021 flu season the following people are eligible to receive the flu vaccine. Such vaccines normally contain a strain of bird flu virus for example AH5N1 that few people in the world have already been exposed to and that could potentially cause a pandemic.
Inactivated influenza vaccines IIV are administered by injection.

Contemporary H3n2 Influenza Viruses Have A Glycosylation Site That Alters Binding Of Antibodies Elicited By Egg Adapted Vaccine Strains Pnas

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram

Naci Abbreviations For Influenza Vaccines Download Scientific Diagram

Difference Between Inactivated And Recombinant Flu Vaccine Compare The Difference Between Similar Terms

Quadrivalent Inactivated Influenza Vaccine Ip Packaging Type Box For Clinic Rs 850 Unit Id 20562973030
Influenza Optimizing Prevention And Treatment Strategies
A Comprehensive Overview Of Vaccines Developed For Pandemic Viral Pathogens Over The Past Two Decades Including Those In Clinical Trials For The Curre Rsc Advances Rsc Publishing Doi 10 1039 D0ra09668g

Influenza Vaccine Effectiveness By Control Group And Vaccine Type Download Table
Atagi Advice On Seasonal Influenza Vaccines In 2020 Australian Government Department Of Health

Canadian Immunization Guide Chapter On Influenza And Statement On Seasonal Influenza Vaccine For 2021 2022 Canada Ca

Facts And Statistics About The Flu
World Health Organization Who Q Do Vaccines Against Pneumonia Protect You Against The New Coronavirus A No Vaccines Against Pneumonia Such As Pneumococcal Vaccine And Haemophilus Influenza Type B Hib Vaccine
H 1 N 1 Influenza Vaccine Ana Mara

Influenza Vaccine 2021 Be Well

7 Types Of Influenza Vaccine The Vaccines Produced To Combat Influenza Download Scientific Diagram
Vaccines Free Full Text Egg Independent Influenza Vaccines And Vaccine Candidates Html
Take These Steps To Improve Your Flu Season Preparedness Mdedge Family Medicine

0 Response to "Influenza Vaccine Type"
Post a Comment